Answer:
The glass bottle will get crack.
Step-by-step explanation:
Volume of the glass bottles,V = 250 mL
Density of water at 20°C =
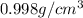
Mass of water in bottle = M
Volume of water at 20°C ,V' = 250 mL (
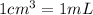 )
)
V>V'
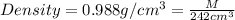
M = 239.096 g
Since mass remains constant, the mass of liquid water will be equal to mass of water when freezes
Density of ice at -5°C =
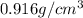
Volume of the ice at -5°C= V''
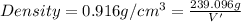
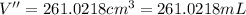
V''>V>V'
As we can see that volume of water has expanded on freezing and exceeded the volume of the glass bottle. This expansion will result in breaking or cracking of a glass bottle.