Answer:
![([A^-])/([HA])=1](https://img.qammunity.org/2018/formulas/chemistry/college/zg7p9hvltardlujzrbw7cjoeq4r9mt81w0.png)
Step-by-step explanation:
Hello,
In this case, one state the following relationship among the pH, pKa and the [a–]/[ha] ratio for the formic acid:
![([A^-])/([HA])=(Ka)/([H^+])](https://img.qammunity.org/2018/formulas/chemistry/college/zb01ha3kgrmn8b00lyx29fevjp82qa12k6.png)
In such a way, we compute both the concentration of hydrogen ions and the acid's dissociation constant as:
![[H^+]=10^(-pH)=10^(-3.75)=1.78x10^(-4)M](https://img.qammunity.org/2018/formulas/chemistry/college/qc75w3463ugc5aklqj2ho9cdchbgrrwhab.png)
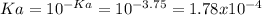
Thus, the [a–]/[ha] ratio becomes:
![([A^-])/([HA])=(1.78x10^(-4))/(1.78x10^(-4))\\([A^-])/([HA])=1](https://img.qammunity.org/2018/formulas/chemistry/college/l7v7r2t9ewjmmw1timp2em5kytcyznp2wq.png)
Best regards.