Step-by-step explanation:
When an atom gain electrons then it acquires a negative charge whereas when an atom loses electrons then it acquires a positive charge.
For example, atomic number of bromine is 35 and its electronic distribution is 2, 8, 18, 7. So, in order to gain stability bromine accepts one electrons and thus it acquires a negative charge as
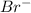 .
.
Therefore, the symbol for the ion formed when each given element gains electrons and attains a noble-gas electron configuration are as follows.
- Br changes into
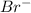 .
.
- H changes into
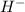 .
.
- Se changes into
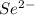 .
.