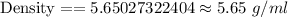Answer: 5.65 g/ml
Explanation:
The density of a substance is given by :-
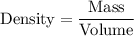
Given : The mass of a metal is 10.34 g. Its volume is 1.83 ml.
Then, the density of substance will be :-
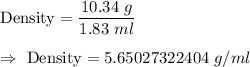
Since , the significant figures depend on the dividend .
Here the dividend (10.34) is hundredth unit.
Then, the