Answer:
Density of Solid A is greater than B
Step-by-step explanation:
Given:
Mass of Solid A = 2 g
Mass of solid B = 0.5 g
Volume of both solids = 6 cm3
To determine:
Densities of A and B
Calculation:
Density of a substance is the mass occupied by it in unit volume
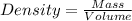
For Solid A:
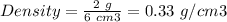
For Solid B :
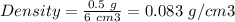
Therefore solid A is more dense than solid B