Answer:
Chemical change
Compounds
Number of atoms of hydrogen on both side=6
Number of atoms of nitrogen on both sides=2
Number of atoms of nitrogen and hydrogen on both sides are equal.
Step-by-step explanation:
We are given that a reaction
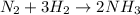
It is chemical change because there is chemical reaction occurring .In physical change, no chemical reaction takes place.
It involved compound because compound consist of two or more than two atoms of same or different elements . In compound , the atoms are bonded together by chemical bond.
Nitrogen is a compound because two nitrogen atoms are bonded together to form a molecule of nitrogen.Similarly , in hydrogen molecules, two hydrogen atom are bonded together to form one hydrogen molecule.
In ammonia, 3 hydrogen and 1 nitrogen atom are bonded together to form ammonia.
On LHS
Number of atoms of hydrogen=6
Number of nitrogen atoms=2
On RHS
Number of atoms of Hydrogen=6
Number of nitrogen atoms=2
Number of atoms of every element in reactants equals to number of atoms of every element in product.