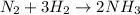Answer: Option (d) is the correct answer.
Step-by-step explanation:
When nitrogen gas chemically reacts with hydrogen gas then it results in the formation of ammonia gas.
The chemical reaction equation for this will be as follows.
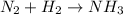
To balance this equation, we need to a multiply
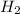 by 3 on reactant side, And, we also need to multiply
by 3 on reactant side, And, we also need to multiply
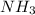 by 2.
by 2.
Therefore, the equation which will correctly represent this reaction will be as follows.