Answer: 5566 years
Explanation:
We know that the half-life of carbon-14 is 5730 years.
Let x be the original amount of C-14 , then the current amount of C-14 =
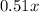
The radioactive half-life formula for C-14 is given by :-
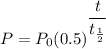 , where P is the amount of C-14 at time t and
, where P is the amount of C-14 at time t and
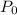 is the original amount.
is the original amount.
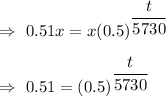
Taking log on both the sides , we get
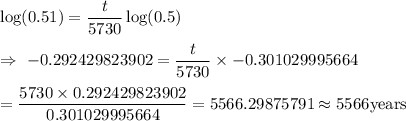
Hence, the age of the spear head is 5566 years.