Answer:
B) The theoretical yield of Cl2 for the reaction is 61.4505 grams
C) The percentage yield of the reaction is 93.57%
Explanations:
B) Given the reaction between HCl and Oxygen expressed as:
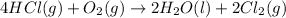
Calculate the moles of HCl;
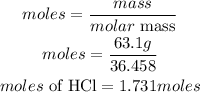
Calculate the moles of Oxygen
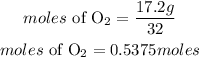
Since there are 4 moles of HCl, hence the limiting reactant will be HCl
According to stochiometry, you can see that 4 moles of HCl produces 2 mole of Cl2, hence the moles of Cl2 produced will be;
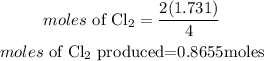
Determin the mass of Cl2 (theoretical yield)
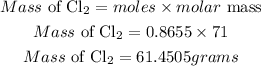
Hence the theoretical yield of Cl2 for the reaction is 61.4505 grams
C) Find the percentage yield for the reaction:

Hence the percentage yield of the reaction is 93.57%