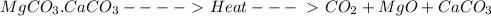
Now, question is why MgO but not CaO.
At a dissociation pressure of 1 atm, the temperature at which CO2 and metal oxide is formed is :
For MgCO3 = 540 °C
For CaCO3 = 900 °C”
Also,
The driving force is the larger lattice energy of MgO(3795) vs that of CaO(3414 kJ mol^-1) . More the lattice energy, the more chances of that compound to form.
So MgO is formed first.
Carbonate is Calcium carbonate