Answer: The mass of mercury is 34000 grams.
Step-by-step explanation:
To calculate mass of a substance, we use the equation:
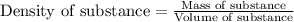
We are given:
Density of mercury =
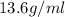
Volume of mercury =
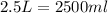
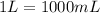
Putting values in above equation, we get:
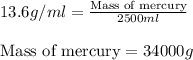
Hence, the mass of mercury is 34000 grams.