Answer: Option (d) is the correct answer.
Step-by-step explanation:
The reaction given is as follows.
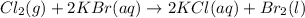
From this equation, it can be seen that chlorine gas reacts with aqueous potassium bromide solution to give aqueous potassium chloride and liquid bromine.
Also, chlorine is more reactive than bromine because it is smaller in size than bromine and has less shielding effect so it will readily accept an electron. Thus, chlorine displaces bromine from potassium bromide solution.
Thus, we can conclude that chlorine gas reacts with potassium bromide to form potassium chloride in solution and liquid bromine.