Answer:
23 J/g
Step-by-step explanation:
The heat transferred in a phase change like this case is calculated as:
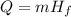
Where m is the mass and Hf is the latent heat of fusion. We can solve the equation for Hf
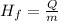
Now, replacing Q = 2.99 x 10⁴ J and the mass = 1300 g, we get:
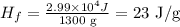
We use 1300 g instead of 1.30 kg because we want the units in J/g.
Therefore, the latent hear of fusion of this metal is 23 J/g