Answer
34.40 grams of H₂O are made.
Step-by-step explanation
The first step is to balance the chemical equation for the reaction:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
From the balanced equation;
1 mole of C₃H₈ produced 4 moles of H₂O
1 mole of C₃H₈ = 44.1 g/mol
1 mole of H₂O = 18.015 g/mol
This implies;
(1 mol x 44.1 g/mol) = 44.1 g of C₃H₈ produced (4 mol x 18.015 g/mol) = 72.06 of H₂O
Therefore, 23.5 g of C₃H₈ will produce
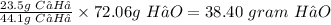
Hence, 34.40 grams of H₂O are made