Answer: 2mg
Part per million is unit that equal to mg/kg of water or simply
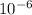
. In this case, you asked how much NaCl needed and know the volume of water(100mL), concentration(20ppm or 20 mg/kg) and water density.
Then, the equation would be
concentration = NaCl weight/ water weight
20 x
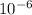
mg= NaCl weight/ (100ml x 1g/ml)
NaCl weight= 20x
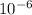
mg/kg x 100g
NaCl weight= 2000 x
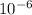
g
NaCl weight= 2x
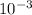
g = 2mg