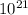Answer: 2.21 x
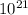
molecule
To answer this question, you need to know the formaldehyde molecular mass.
Formaldehyde molecular weight would be 12+ 1(2) + 16= 30 g/mol
After knowing the molecular mass you can count how many molecules by multiplying it by Avogadro's number. The calculation would be:
0.11g / (30g/mol) x 6.022140857 ×
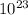
= 0.02208 x
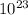
= 2.21 x