Answer:
a)109° b)180° c)120°
Step-by-step explanation:
Hi, in general the bond angle in any molecule depends of:
- The type of bond (single, double, triple)
- The presence of electron pairs the central atom.
In particular for C atoms, there isn't free electron pairs so the angle only depends of the type of bond.
a) Propanol (
 )
)
Is this molecule all C have single bonds and an hibridization of sp3, forming a tetrehedral geometry. The bond angle determined for this geometry is arround 109°
b)2-Butyne (
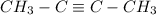 )
)
Is this molecule, the triple bond makes a sp hibridization, forming a linear geometry. The bond angle determined for this geometry is 180°
a) Propadiene (
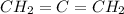 )
)
Because of the double bonds, C have sp2 hibridization, forming a plain geometry. The bond angle determined for this geometry is 120°