Answer:
Nitric acid is an oxidizing agent in the given reaction.
Step-by-step explanation:
Oxidizing agents : Agents which oxidize other substance and itself gets reduced. These agents undergoes reduction reactions.
Reducing agents : Agents which reduces the other substance and itself gets oxidized. These agents undergoes reduction reactions.
In the given reaction;
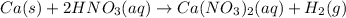
Oxidizing agent in the above reaction is a nitric acid which oxidizes calcium metal into calcium ion
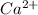
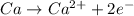
Here calcium atom is undergoing oxidation reaction which is the reaction in which an atom looses its electrons and its oxidation state increases.