Step-by-step explanation
Given:
Molar mass of ascorbic acid, M = 176 g/mol
Moles of ascorbic acid in sweet lime = 2.88 x 10⁴ mol
what to find:
The mass of ascorbic acid in 2.88 x 10⁴ mol of ascorbic acid.
Step-by-step solution:
The relationship between mole, n, reacting mass, m, and molar mass, M, is given by
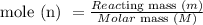
So, substitute the given moles and molar mass of ascorbic acid into the formula above to get the mass of ascorbic acid:
![undefined]()