Answer:
18
Step-by-step explanation:
The mass number of an isotope could be calculated using the following equation;
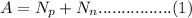
where A represents the mass number,
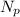 represents the number of protons and
represents the number of protons and
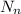 represents the number of electrons.
represents the number of electrons.
Given;
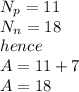
isotopes are defined generally as two or more atoms of the same element having the same atomic number but different mass numbers.
Hence the property that differs in isotopes is their mass numbers.