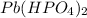Answer:
a)
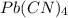
b)
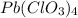
c)
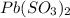
d)
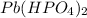
Step-by-step explanation:
In the given scenario, Pb⁴⁺ is a cation which combines with various anions in order to form a neutral ionic compound. This implies that the charges on the cation and anion must be balanced so that the net charge on the molecule is zero.
a) Pb⁴⁺ with CN⁻
Charge on Pb = +4
Charge on CN = -1
To form a neutral molecule, each Pb⁴⁺ should combine with 4 CN⁻
Formula:
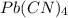
b) Pb⁴⁺ with ClO₃⁻
Charge on Pb = +4
Charge on ClO₃⁻ = -1
To form a neutral molecule, each Pb⁴⁺ should combine with 4 ClO₃⁻
Formula:
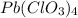
c) Pb⁴⁺ with SO₃²⁻
Charge on Pb = +4
Charge on SO₃²⁻ = -2
To form a neutral molecule, each Pb⁴⁺ should combine with 4 ClO₃⁻
Formula:
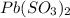
d) Pb⁴⁺ with HPO₄²⁻
Charge on Pb = +4
Charge on HPO₄²⁻ = -2
To form a neutral molecule, each Pb⁴⁺ should combine with 4 ClO₃⁻
Formula: