Answer: The rate of second reaction is
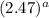 times the rate of the first reaction.
times the rate of the first reaction.
Step-by-step explanation:

For the first reaction where concentration o the reactant is [A]= 0.230 mol/L
![R_1=k* [A]^a=k* [0.230 mol/L]^a](https://img.qammunity.org/2018/formulas/chemistry/high-school/om6s6h2ww2nlcfwlbsr9ziyn82tqmj6m3p.png) ..(1)
..(1)
For the second reaction where concentration o the reactant is [A]= 0.570 mol/L
{tex]R_2=k\times [A]^a=k\times [0.570 mol/L]^a[/tex]...(2)
Dividing (2) and (1)
![(R_2)/(R_1)=(k* [0.570 mol/L]^a)/(k* [0.230 mol/L]^a)](https://img.qammunity.org/2018/formulas/chemistry/high-school/5r1is9pp74whwfu9bf4m0ecmvl9pwdzeez.png)
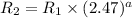
The rate of second reaction is
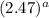 times the rate of the first reaction.
times the rate of the first reaction.