Answer:
Density of the gold nugget,
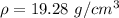
Step-by-step explanation:
It is given that,
Mass of the gold nugget, m = 32.4 g
Volume of the gold nugget
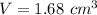
We need to find the density of the gold nugget. The total mass divided by total volume of an substance is called its density. Its formula is given by :
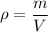
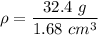
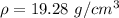
So, the density of the metal ball is
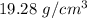 . Hence, this is the required solution.
. Hence, this is the required solution.