Answer:- An empirical formula describes the type and ratio of atoms present in a chemical compound.
For example, chemical formula for benzene is
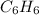 . It has six carbons and six hydrogens. The ratio of C to H atoms is 1:1 and so the empirical formula of benzene is CH.
. It has six carbons and six hydrogens. The ratio of C to H atoms is 1:1 and so the empirical formula of benzene is CH.