Answer : The correct option is, 54.6 %
Explanation: Given,
Mass of C = 98.28 g
Mass of H = 16.21 g
Mass of O = 65.52 g
First we have to calculate the total mass of the compound.
Total mass of compound = Mass of C + Mass of H + Mass of O
Total mass of compound = 98.28 + 16.21 + 65.52 = 180.01 g
Now we have to calculate the percent composition of carbon in the compound.
Formula used :
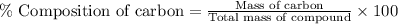
Now put all the given values in this formula, we get the percent composition of carbon in the compound.
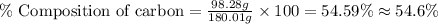
Therefore, the percent composition of carbon in the compound is, 54.6 %