Answer: 0.018 M
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
Acids have pH ranging from 1 to 6.9, bases have pH ranging from 7.1 to 14 and neutral solutions have pH equal to 7.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
Given: pH= 12.25
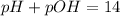
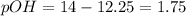
![pOH=-log[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/wg3zegzrhdkew96s1c2vjux38xrb1fgg4y.png)
![1.75=-log[OH^-]](https://img.qammunity.org/2018/formulas/chemistry/high-school/s6tiwb28agus2filgckqd8dfjihqobm8ss.png)
![[OH^-]=0.018M](https://img.qammunity.org/2018/formulas/chemistry/high-school/ucdc0defxlj1l18blmo4mzzi55fqw2xie8.png)
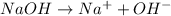
Thus as 1 mole of
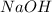 gives 1 mole of
gives 1 mole of
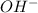 thus concentration of
thus concentration of
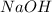 will be 0.018M.
will be 0.018M.