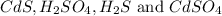Answer: The chemical species are
Step-by-step explanation:
When cadmium sulfide reacts with sulfuric acid, it leads to the production of hydrogen sulfide and cadmium sulfate.
The chemical equation for the reaction of cadmium sulfide and sulfuric acid follows:
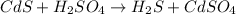
This is a double displacement reaction because here, exchange of ions are taking place.
Hence, the chemical species are