Answer:
all the possible masses of
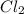 molecules that are made when two chlorine atoms bond together are 70,72 and 74
molecules that are made when two chlorine atoms bond together are 70,72 and 74
Step-by-step explanation:
Chlorine (Cl) has two most common known isotopes these are as follows
Cl-35 and and Cl-37. It means Cl has two possible masses these are 35 and 37.
all the possible masses of
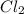 molecules that are made when two chlorine atoms bond together are as follows
molecules that are made when two chlorine atoms bond together are as follows
it is possible that both the atom in
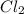 are Cl-35 then atomic mass of
are Cl-35 then atomic mass of
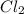 will be
will be
35 + 35 = 70
it is possible that both the atom in
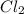 are Cl-37 then atomic mass of
are Cl-37 then atomic mass of
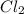 will be
will be
37 + 37 = 74
it is possible that
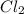 is formed when Cl-37 and Cl-35 are bond together, then atomic mass of
is formed when Cl-37 and Cl-35 are bond together, then atomic mass of
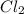 will be
will be
35 + 37 = 72