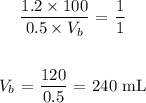Answer:
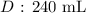
Step-by-step explanation:
We start by writing the equation of the reaction
We have this as:
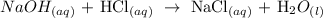
Now, we proceed to write the equation for standardization. We have this as:
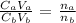
where:
Ca is the molarity of HCl which is 1.2M
Cb is the molarity of NaOH which is 0.50M
Va is the volume of the acid which is 100mL
Vb is the volume of the base which is what we want to calculate
na is the number of moles of the acid which is 1 (as seen from the balanced equation of neutralization)
nb is the number of moles of the base which is 1
Substituting the values, we have it that: