Answer:
Density,
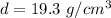
Step-by-step explanation:
Mass of the sample of gold, m = 38.6 grams
Volume of gold sample,
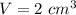
The density of gold sample is given by mass per unit volume. Mathematically, it is given by :
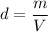
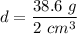
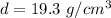
So, the density of the sample of gold is
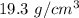 . Hence, this is the required solution.
. Hence, this is the required solution.