Answer:
The correct answer is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Step-by-step explanation:
When aqueous solution of hydrogen chloride reacts with aqueous solution of sodium hydroxide to give aqueous solution of sodium chloride and water.
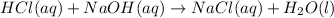
1 mole of hydrogen chloride reacts with 1 mol of sodium hydroxide to give 1 mole of sodium chloride and 1 mole of water.