Answer: Option (b) is the correct answer.
Step-by-step explanation:
When calcium carbonate is added to an acid then the reaction will be as follows.
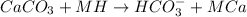
The
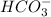 formed is easily soluble in water.
formed is easily soluble in water.
Therefore, when we crush calcium carbonate the surface area of particles will increase. As a result more number of molecules will be dispersed in the acid, hence more is the number of calcium carbonate particles more readily the reaction will occur.
Thus, we can conclude that the rate of this reaction increases when we crush the lime stone or calcium carbonate.