Answer:
The strength of the sucrose solution is 65.03624 g/L.
Molality of the solution is 0.1989 mol/kg.
Mass percentage of the solution is 6.37%
Step-by-step explanation:
Strength of the sucrose solution
Molarity of the sucrose solution = 0.190 M = 0.190 mol/L
Molar mass of sucrose = 342.296 g/mol
Strength of the solution = Molarity of the solution × Molar mass of the solute
Strength of the sucrose solution =
=
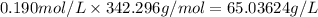
Molality of the solution:
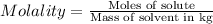
Moles of sucrose in 1L solution = 0.190 moles
Mass of water = 955.0 g = 0.9550 kg
Molality of the solution is:
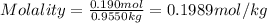
Mass percentage of the solution:
Density iof the solution =
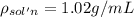
Mass of solution = Mass of solute + Mass of water
Volume of solution = 1L = 1,000 mL
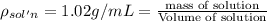
Mass of solution = 1.02 g/mL × 1,000 mL = 1,020 g
Mass of solute + Mass of water = 1,020 g
Mass of solute = 1,020 g - 955.0 g = 65.0 g
Mass percentage:
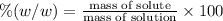
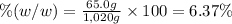
Mass percentage of the solution is 6.37%