Answer: The balanced chemical reaction is given as:
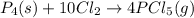
Step-by-step explanation:
The equation for the reaction of phosphorous and chlorine gas is given by:
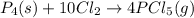
By stoichiometry of the reaction:
2 moles of phosphorous reacts with 5 moles of chlorine gas gives 2 moles of phosphorus pentachloride gas as a product.