Given
Number of moles, n=4 mol
The temperature is T=290K
Mass of the gas, m=0.064 kg
Universal gas constant, R=8.31 J/mol.K
To find
The average speed of the molecule
Step-by-step explanation
The average speed is given by
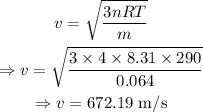
Conclusion
The average speed is B. 672 m/s