Answer : The correct option is, (c)
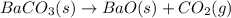
Explanation :
Decomposition reaction : It is a type of chemical reaction in which one reactant breaks into two or more smaller compounds.
The given balanced decomposition reaction of barium carbonate is,
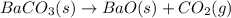
In this reaction, 1 mole of barium carbonate decomposes to yields 1 mole of barium oxide and 1 mole of carbon dioxide.