Answer: 0.25 grams per milliliter
Step-by-step explanation:
The formula to calculate the density is given by :-
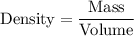
We are given that the volume of liquid is 24.0 mL.
Mass = 6g
Now, the density of liquid is given by :-
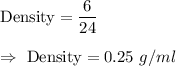
Hence, its density = 0.25 grams per milliliter.