Given:
The heat content of the pizza is Q = 500 kcal
The volume of the cold water is V = 50 L
The mass of a liter of cold water is 1 kg.
Calculate the mass, M, of cold water.
M = (50 L)*(1 kg/L) = 50 kg
The specific heat of water is
c = 4.184 kJ/(kg-K)
Also,
1 kcal = 4184 J, therefore
Q = (500 kcal)*(4184 J/kcal) = 2.092 x 10⁶ J
Let ΔT = increase in temperature of the cold water, °C (same as K).
Then
Q = M*c*ΔT
or
ΔT = Q/(M*c)
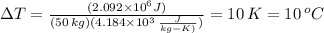
Answer: The temperature increases by 10 °C (or 10 K)