1) Heat absorbed by water
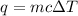
q= heat absorbed
m= mass (grams)
c=specific heat of water is 4.18 J/g-°C
ΔT= final temperature - initial temperature
2) Plug in known values
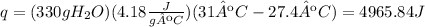
3) Heat transferred.
Heat absorbed by water is the heat released by the sample.
4) Heat transfer by sample
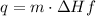
q= heat transfer
m= mass (grams)
ΔHf=enthalpy of fusion
5) Plug in known values and solve for ΔHf.
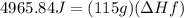
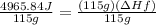
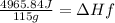
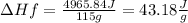
The enthalpy of fusion of the sample is 43.18J/g.