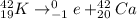In the 1st reaction, ⁴²K undergoes beta decay. Therefore the resulting element will have 1 more proton than ⁴²K.
Therefore, the mass number of the new particle is 42.
The atomic number of potassium is 19.
Therefore the atomic number of the new element will be 19+1=20.
Therefore the new element is Calcium.
Therefore the reaction is,