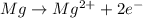Answer: Magnesium must lose two electrons to become
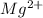
Explanation: Magnesium(Mg) ia a metal with atomic number of 12 and atomic mass of 24.
Atomic no = no of electrons = no of protons
Atomic Mass = no. of protons + no. of neutrons
Thus Magnesium in neutral form has got 12 electrons and 12 protons. In order to acquire a charge of +2 it must loose two electrons. Now the no of protons will be greater than no of electrons and thus the atom will acquire positive charge.