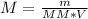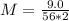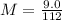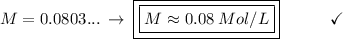Data:
M (molarity) = ? (Mol/L)
m (mass) = ? (in grams)
V (volume) = 2 L
MM (Molar Mass) of Potassium Hydroxide (KOH)
K = 1*39 = 39 amu
O = 1*16 = 16 amu
H = 1*1 = 1 amu
------------------------------------
MM of KOH = 39+16+1 = 56 g/mol
***
Let's find the mass
Data:
n (number of mols) = 4.5 mols
m (mass) = ? (in grams)
v (volume) = 2 L
Formula:
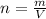
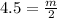
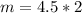
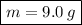
***Let's find the molarity:
Formula:
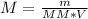
Solving: