Balance the equation first:
2 KClO3 (s) ---> 2 KCl (s) + 3 O2 (g)
Moles of KClO3 = 110 / 122.5 = 0.89
Following the balanced chemical equation:
We can say moles of O2 produce =
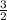
x moles of KClO3
So, O2 = (3 / 2) x 0.89
= 1.34 moles
So, Volume at STP = nRT / P
T =
273.15 K
P = 1 atm
So, V = (1.34 x 0.0821 x 273.15) / 1 = 30.2 L