Answer:
D. 36 x 1023 molecules of Na2O.
Step-by-step explanation:
1st) According to the balanced reaction, 4 moles of Na react with 1 mole of O2 to produce 2 moles of Na2O. Using the molar mass of sodium (23g/mol), we can convert moles into grams:
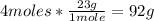
Now we know that from 92g of sodium, 2 moles of Na2O are produced.
2nd) We can calculate the moles of Na2O that will be created using 275g of Na and excess oxygen, with a mathematical rule of three:
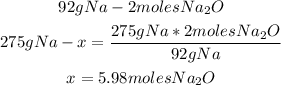
Now we know that from 275g of Na, 5.98 moles of Na2O are created.
3rd) Finally, we can convert the 5.98 moles of Na2O into molecules using the Avogadro's number (6.022x10^23 particles/mol):
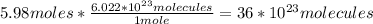
So, 36x10^23 molecules are created.