Answer: Option (a) is the correct answer.
Step-by-step explanation:
It is known that density is the amount of mass divided by volume.
Mathematically, Density =
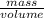
It is given that density is 8.90
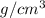 and mass is 798 g.
and mass is 798 g.
Therefore, calculate the volume as follows.
Density =
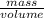
8.90
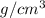 =
=
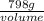
volume = 89.7
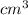
As 1
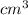 = 1 ml. Hence, volume is 89.7 ml.
= 1 ml. Hence, volume is 89.7 ml.
Thus, we can conclude that if a sample of copper with a density of 8.90 g/cm3 has a mass of 798 g then its volume is 89.7 ml.