Answer: The correct answer is option (A).
Explanation:
General representation of an element:
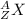
A= Atomic mass of the element of X.
Z = Atomic number of the element of X
X = symbol of the element.
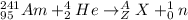
According to Law of Conservation of Mass:
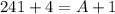
A = 244
Charge is balanced on both the sides:
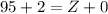
Z = 97
The element with the atomic number 97 is Berkelium. Hence, the correct answer is option (A).