Answer:
80.44 grams
Explanation:
We have to calculate the weight ( in grams ) of a liquid that exactly fills a 163.5 milliliter container if the density of the liquid is 0.492 grams over milliliter.
The formula to find density
ρ
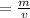
ρ = Density
m = mass
v = volume
To find mass (weight), we will rewrite the equation in terms of m
m = ρ × v
m = 163.5 × (0.492g/ml)
m = 80.442 grams ≈ 80.44 grams
The weight of a liquid is 80.44 grams that fills a container.