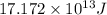Answer: The binding energy of one mole of thorium atom is
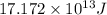
Step-by-step explanation:
Binding energy is defined as the energy which holds the nucleus together. It is basically the product of mass defect and the square of the speed of light.
This energy is calculated by using Einstein's equation, which is:

Where,
E = Binding energy of the atom
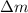 = Mass defect = 1.908 g/mol =
= Mass defect = 1.908 g/mol =
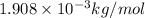 (Conversion factor: 1 kg = 1000 g)
(Conversion factor: 1 kg = 1000 g)
c = speed of light =
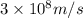
Putting values in above equation, we get:
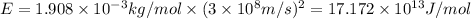
Hence, the binding energy of one mole of thorium atom is