Answer:
15 moles of H2.
Step-by-step explanation:
First, let's write the chemical reaction:
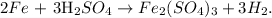
Now, we can find how many moles of H2 are being produced by 10 moles of Fe doing a rule of three. You can see in the chemical equation that 2 moles of Fe reacted produces 3 moles of H2, so the rule of three would look like this:
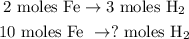
And the calculation would be:
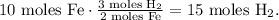
We will expect to produce 15 moles of H2.