Answer:
15 moles
Step-by-step explanation:
According to Avogadro’s number 1 mole of a substance has 6.023 x 1023 particles.
Particles could be atoms, molecules, ions, formula unit etc. Avogadro’s number allows scientists to manipulate very large number as real life material contain very large numbers of particles such as atoms, molecules, ions etc.
9.03 x 10²⁴ x 1 mole/
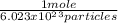 = 15 moles
= 15 moles